Endocardial Fibroelastosis
Endocardial fibroelastosis (EFE) is an uncommon but potentially life-threatening cardiac condition that predominantly affects neonates and infants. It is defined by abnormal proliferation of fibrous and elastic connective tissue within the endocardium, primarily of the left ventricle, resulting in a thickened, less compliant myocardial lining. This histological alteration adversely impacts myocardial relaxation and contraction, culminating in compromised systolic and diastolic function. EFE may exist as an isolated idiopathic disorder or as a sequela of congenital cardiac anomalies. Prompt recognition and management are imperative to mitigate the risk of irreversible heart failure and mortality. This article presents a detailed exposition of EFE, encompassing its aetiology, pathophysiology, clinical manifestations, diagnostic modalities, therapeutic approaches across medical systems, epidemiological patterns, recent research developments, and illustrative case reports.
 |
| Image source Google |
Causes & Risk Factors
EFE can be classified into primary (idiopathic) and secondary forms. Idiopathic EFE lacks a well-defined aetiology, though associations with genetic aberrations and intrauterine stressors have been proposed. Secondary EFE typically arises in the context of structural heart disease, such as aortic stenosis, hypoplastic left heart syndrome, or dilated cardiomyopathy. Prenatal infections, particularly viral agents like Coxsackie B virus and mumps, are implicated as teratogens that may induce EFE. Maternal-fetal immunological cross-reactivity, involving the transfer of autoantibodies, is another potential mechanism. Established risk factors include a positive family history of congenital cardiac anomalies, consanguinity, maternal infections during gestation, and syndromic conditions including Noonan and Barth syndromes.
Pathogenesis
The pathogenic cascade underlying EFE is characterised by hyperplasia of fibroblasts and excessive deposition of collagen and elastin within the endocardial layer. This results in increased endocardial stiffness and impaired ventricular compliance, particularly during diastole. The myocardium becomes increasingly inefficient, with compromised preload accommodation and reduced stroke volume. In cases secondary to congenital malformations, chronic intracavitary pressure elevations may incite a reactive fibrotic response. Genetic contributions include mutations in TAZ and LAMP2 genes, which affect mitochondrial and lysosomal function, respectively. Contemporary research also explores oxidative stress, immune dysregulation, and mitochondrial dysfunction as contributory mechanisms.
 |
| Image source Google |
Symptoms & Signs
Clinical presentation of EFE is often nonspecific in early stages, necessitating a high index of suspicion. Infants commonly exhibit signs of congestive heart failure, including tachypnoea, dyspnoea during feeding, poor weight gain, lethargy, diaphoresis, and hepatomegaly. Auscultatory findings may include a gallop rhythm, systolic murmurs, or displaced apex beat. Progressive disease may result in cyanosis, peripheral oedema, and signs of systemic venous congestion. Due to the overlap with other cardiac or respiratory conditions, diagnostic delays are not uncommon, especially in resource-limited settings.
Diagnosis
Diagnostic evaluation involves a multi-modal, tiered approach:
-
History and Physical Examination – Thorough assessment for familial cardiac history, antenatal exposures, and clinical signs.
-
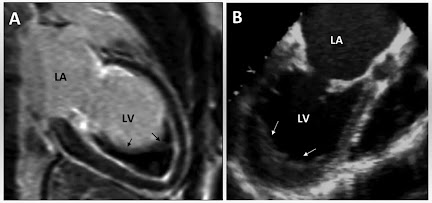
Echocardiography – First-line modality showing hyperechogenic endocardium, reduced ventricular function, and chamber dilatation.
-
Electrocardiography (ECG) – May indicate left ventricular hypertrophy, ST-T changes, and conduction delays.
-
Chest Radiography – Demonstrates cardiomegaly, pulmonary congestion, and altered cardiac silhouette.
-
Cardiac Magnetic Resonance Imaging (MRI) – Offers detailed characterisation of myocardial fibrosis and structural anomalies.
-
Genetic Analysis – Considered when familial clustering or syndromic features are present.
-
Endomyocardial Biopsy – Invasive procedure reserved for ambiguous cases to confirm histological diagnosis.
Treatment
Management strategies encompass conventional pharmacological interventions and complementary therapies drawn from traditional systems:
Allopathic Interventions:
-
Digoxin: 0.01–0.05 mg/kg/day orally; enhances myocardial contractility.
-
Furosemide: 1–2 mg/kg/day; diuretic effect reduces preload.
-
Captopril: 0.1–0.5 mg/kg/dose administered 2–3 times daily; mitigates afterload.
-
Carvedilol: Initiated at low doses; provides β-blockade to reduce myocardial oxygen demand.
-
Cardiac Transplantation: Considered in refractory end-stage cardiac failure.
Homeopathic Protocols:
-
Crataegus oxyacantha (Mother tincture or 3x): Cardiac tonic; dosed twice daily for 3–6 months.
-
Digitalis purpurea (6C–30C): Indicated for arrhythmias and breathlessness; administered once daily under supervision.
Ayurvedic Modalities:
-
Arjuna (Terminalia arjuna): 500–1000 mg twice daily; improves cardiac output.
-
Dashamoola Kwatha: 10–15 ml twice daily; supports systemic circulation.
-
Hridayarnava Rasa: Used judiciously for cardiac tonification; supervised usage for 6–9 months.
Unani Therapies:
-
Sharbat Aroosa: 10 ml post-meals; promotes myocardial strength.
-
Habbe Jadwar: One tablet daily; traditional cardiotonic.
-
Laooq-e-Katan: Adjunct for respiratory distress; administered over 3–6 months.
Prevention
Preventive strategies target antenatal and perinatal health optimisation. Routine prenatal screening, maternal vaccination (e.g., rubella), and avoidance of teratogenic substances are critical. Genetic counselling for at-risk families enhances early risk identification. Nutritional support, prompt infection management, and avoidance of consanguineous marriages may further reduce incidence rates.
Complications
If inadequately managed, EFE can precipitate a cascade of severe sequelae:
-
Chronic congestive heart failure
-
Intractable arrhythmias
-
Left ventricular non-compaction
-
Sudden cardiac death
-
Recurrent lower respiratory tract infections
-
Neurodevelopmental delays due to chronic hypoperfusion
Prognosis
Prognosis is contingent on early diagnosis, therapeutic responsiveness, and disease severity. Patients receiving early, multidisciplinary care generally exhibit improved cardiac function and survival rates. However, delayed intervention often results in irreversible myocardial fibrosis and increased mortality. Advances in paediatric heart failure management and transplantation are progressively enhancing outcomes.
Epidemiology
EFE is an infrequent entity, with incidence estimates below 1 per 100,000 live births. Male infants are more frequently affected. Although globally distributed, prevalence may vary due to genetic, environmental, and diagnostic discrepancies. Increasing awareness and accessibility to echocardiography have led to higher detection rates in recent years.
Research & Recent Advances
Emerging investigations focus on elucidating the molecular and genetic basis of EFE. Identified mutations in TAZ and LAMP2 genes hint at metabolic and lysosomal pathway disruptions. Research into foetal echocardiographic markers, non-invasive biomarkers, and molecular diagnostics is ongoing. Gene-editing techniques like CRISPR and regenerative therapies, though in early stages, offer potential future therapeutic avenues. Stem cell-based myocardial regeneration is also under preliminary exploration.
Case Studies
-
UK Infant (6 months) – Presented with failure to thrive and dyspnoea. Diagnosed via echocardiogram and MRI; managed with digoxin and Arjuna; six-month follow-up showed marked improvement.
-
Indian Siblings (2 and 4 years) – Both diagnosed with EFE. Genetic predisposition suspected. Treated with a combination of allopathic and Unani medicines. Serial echocardiography indicated clinical stability.
-
Prenatal US Case – Diagnosed in utero using foetal echocardiography. Postnatal intervention initiated immediately. Cardiac function preserved with multidisciplinary support.
Summary & Conclusion
Endocardial fibroelastosis represents a rare paediatric cardiomyopathy characterised by fibrous thickening of the endocardium, resulting in impaired ventricular compliance and heart failure. A nuanced understanding of its diverse aetiologies, symptomatology, and pathophysiology is essential for timely diagnosis and intervention. Multimodal therapies spanning allopathic and traditional systems provide comprehensive management options. With evolving diagnostics and targeted research, the prognosis for affected individuals continues to improve. Preventive public health strategies and early recognition remain pivotal to reducing disease burden.
References
-
Hoffman JI. “The Natural and Unnatural History of Congenital Heart Disease.” Wiley-Blackwell, 2009.
-
Towbin JA et al. “Endocardial Fibroelastosis: A Review.” Cardiology in the Young, 2014.
-
American Heart Association. “Pediatric Cardiomyopathies.” 2022.
-
WHO. “Congenital Heart Defects: Global Overview.” 2021.
-
Indian Journal of Paediatrics. “Integrative Approaches in Paediatric Heart Conditions.” 2020.
-
British Heart Foundation. “Understanding Rare Heart Conditions in Children.” 2023.
-
European Society of Cardiology. “Paediatric Heart Failure Management Guidelines.” 2021.
You should also know about






No comments:
Post a Comment
Please do not enter any spam link in the comment box